Carbonate and carbonate anion radicals in aqueous solutions exist as CO3(H2O)62− and CO3(H2O)6˙− respectively: the crucial role of the inner hydration sphere of anions in explaining their properties†
Abstract
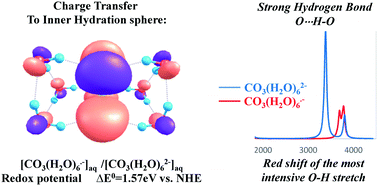
An effort to reproduce the chemical and physical properties of carbonate and carbonate anion radicals in aqueous solutions by DFT proves that one has to include an inner hydration sphere of six water molecules for both anions. Application of the SMD model to CO3(H2O)62− and CO3(H2O)6˙− enables achievement of the experimental value of the redox potential of the CO3(H2O)6˙−/2− couple. This is a result of the direct inclusion of a considerable charge transfer (CT) from CO32− to its inner hydration sphere in the calculation of the hydration effect. The HOMO of clusters is an analogue of the non-bonding σ-type a2′-HOMO of the parent CO3 moiety with a σ*(OH) contribution. This is a MO manifestation of the CT to the first hydration shell. The localization of the CT on the first hydration shell also re-produces the very strong OH⋯O stretch peak. Furthermore, the very large difference in the hydration energies of CO32− and CO3˙− which causes the very large differences in the length of the C–O⋯H–O hydrogen bonds suggests that the oxidations by CO3˙− proceed via the inner sphere mechanism.


 Please wait while we load your content...
Please wait while we load your content...