Insight into vibrational circular dichroism of proteins by density functional modeling†
Abstract
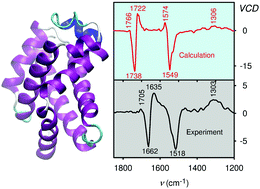
Vibrational circular dichroism (VCD) spectroscopy is an excellent method to determine the secondary structure of proteins in solution. Comparison of experimental spectra with quantum-chemical simulations represents a convenient and objective way to extract information on the structure. This has been difficult for such large molecules where approximate theoretical models have to be used. In the present study we applied the Cartesian-coordinate based tensor transfer (CCT) making it possible to extend the density functional theory (DFT) and model spectral intensities of large globular proteins nearly at quantum-chemical precision. Indeed, comparison with experiment provided a better understanding of the dependence of VCD spectral shapes on the geometry, their sensitivity to fine structural details and interactions with the environment. On a model set of globular proteins the simulated spectra correlated well with experimental data and revealed which structural information can (and cannot) be obtained from this kind of spectroscopy. Although the VCD technique has been regarded as being rather insensitive to side-chain variations, we found that the spectra of human and hen lysozyme differing by a few amino acids only are quite distinct. This has been explained by long-distance coupling of the amide vibrations. Likewise, the modeling reproduced some spectral changes caused by protein deuteration even when the protein structure was conserved.


 Please wait while we load your content...
Please wait while we load your content...