Hydrocarbon decomposition kinetics on the Ir(111) surface
Abstract
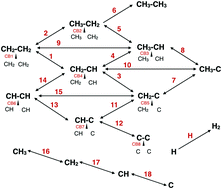
The kinetics of the thermal decomposition of hydrocarbons on the Ir(111) surface is determined using kinetic Monte Carlo (kMC) and rate equations simulations, both based on the density functional theory (DFT) calculated energy barriers of the involved reaction processes. This decomposition process is important for understanding the early stages of epitaxial graphene growth where the deposited hydrocarbon acts as a carbon feedstock for graphene formation. The methodology of the kMC simulations and the rate equation approaches is discussed and a comparison between the results obtained from both approaches is made in the case of the temperature programmed decomposition of ethylene for different initial coverages. The theoretical results are verified against experimental data from in situ X-ray photoelectron spectroscopy (XPS) experiments. Both theoretical approaches give reasonable results; however we find that, as expected, rate equations are less reliable at high coverages. We find that the agreement between experiment and theory can be improved in all cases if slight adjustments are made to the energy barriers in order to account for the intrinsic errors in DFT. Finally we extend our approach to the case where hydrocarbon species are dosed onto the substrate continuously, as in the chemical vapour deposition (CVD) graphene growth method. For ethylene and methane the thermal decomposition mechanism is determined, and it is found that in both cases the formation of C monomers is to be expected, which is limited by the presence of hydrogen atoms.
- This article is part of the themed collection: 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...