Butadiene as a ligand in open sandwich compounds†
Abstract
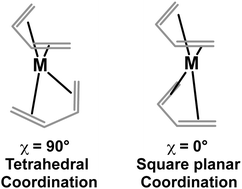
Theoretical methods show that the lowest energy bis(butadiene)metal structures (C4H6)2M (M = Ti to Ni) have a perpendicular relative orientation of the two butadiene ligands corresponding to a tetrahedral coordination of the central metal atom to the four C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds of the butadiene ligands. Distribution of the metal d electrons in the resulting tetrahedral ligand field rationalizes the predicted spin states increasing monotonically from singlet to quartet from nickel to manganese and back from quartet to singlet from manganese to titanium.
C double bonds of the butadiene ligands. Distribution of the metal d electrons in the resulting tetrahedral ligand field rationalizes the predicted spin states increasing monotonically from singlet to quartet from nickel to manganese and back from quartet to singlet from manganese to titanium.


 Please wait while we load your content...
Please wait while we load your content...