State-to-state mode specificity in H + DOH(νOH = 1) → HD + OH(ν2 = 0) reaction: vibrational non-adiabaticity or local-mode excitation?†
Abstract
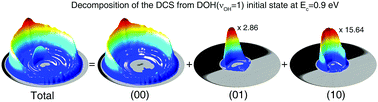
It is well established that chemical reactions often involve only a small number of atoms near the reaction site, and the remainder of the reactant molecules are mostly spectators. It is thus of great importance to understand the role played by the active as well as spectator modes in chemical dynamics. In this work, we examine in great detail the influence of reactant modes on the reactivity and product state distribution, using a four-atom prototypical reaction as the example. State-of-the-art full-dimensional state-to-state quantum dynamics reveal a startling observation in which the DOH(νOH = 1) molecule reacts with a H atom to produce a vibrationless OH product. This is surprising because OH is considered as a spectator in this reaction and its internal energy should be sequestered throughout the reaction. By careful analysis within the local-mode regime, we demonstrate that the surprising reactivity is not due to vibrational non-adiabaticity during the reaction. Rather, it can be attributed to a small OD excited local-mode component in the reactant wavefunction. The quantum state-resolved dissection of this prototype reaction helps to advance our understanding of larger reactive systems.


 Please wait while we load your content...
Please wait while we load your content...