Sumanene: an efficient π-bowl for dihydrogen storage†
Abstract
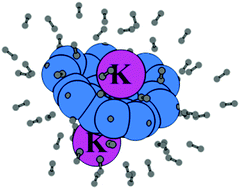
Density functional theory calculations at the M06L/6-311++G(d,p) level show that sumanene (Su), a polycyclic aromatic π-bowl, and its anionic forms possess high dihydrogen binding affinity. The dihydrogen rich systems such as Su(H2)40, Su−(H2)40, and Su2−(H2)40 show interaction energy (Eint) values of 51.7, 63.0 and 87.6 kcal mol−1, respectively. The ion-pair complexes of anionic, dianionic and trianionic sumanenes with K+ also have a significant H2 binding capacity with Eint values of 61.6 kcal mol−1 for Su−K+(H2)47, 77.1 kcal mol−1 for Su2−(K+)2(H2)51 and 132.6 kcal mol−1 for Su3−(K+)3(H2)51. The charge delocalization in the complex increases substantially with an increase in the amount of H2 adsorbed, which parallels with a declining trend in the magnitude of the molecular electrostatic potential (MESP) minimum (Vmin) for Su, Su−, Su2−, Su−K+, Su2−(K+)2, and Su3−(K+)3. Also, using quantum theory of atoms in molecules (QTAIM) analysis, sumanene⋯H2 noncovalent interactions and secondary dihydrogen interactions within the complex are established by locating bond critical points (bcp). The structured network of noncovalent bonds in the complex accounts for the stability of the complex. Further, by replacing K+ with lighter metals such as Li+ or Na+, a 66–74% increase in Eint is observed for anion-M+ and dianion-(M+)2 ion pairs. Our results prove that sumanene systems possess significant dihydrogen binding affinity, which can be employed in developing efficient hydrogen storage systems.


 Please wait while we load your content...
Please wait while we load your content...