The role of charge and proton transfer in fragmentation of hydrogen-bonded nanosystems: the breakup of ammonia clusters upon single photon multi-ionization
Abstract
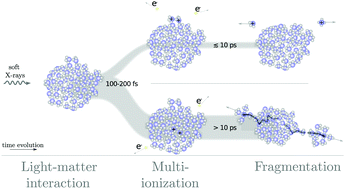
The charge and proton dynamics in hydrogen-bonded networks are investigated using ammonia as a model system. The fragmentation dynamics of medium-sized clusters (1–2 nm) upon single photon multi-ionization is studied, by analyzing the momenta of small ionic fragments. The observed fragmentation pattern of the doubly- and triply-charged clusters reveals a spatial anisotropy of emission between fragments (back-to-back). Protonated fragments exhibit a distinct kinematic correlation, indicating a delay between ionization and fragmentation (fission). The different kinematics observed for channels containing protonated and unprotonated species provides possible insights into the prime mechanisms of charge and proton transfer, as well as proton hopping, in such a nanoscale system.


 Please wait while we load your content...
Please wait while we load your content...