A drastic influence of the anion nature and concentration on high pressure intrusion–extrusion of electrolyte solutions in Silicalite-1†
Abstract
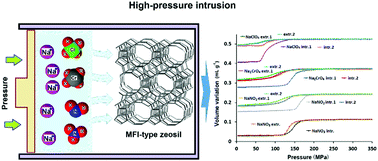
High pressure intrusion–extrusion of concentrated solutions of sodium salts in a pure-silica MFI-type zeolite (Silicalite-1) was studied for potential applications in mechanical energy absorption and storage. It was discovered that the anion nature has a drastic influence on the behavior and the energetic performances of “Silicalite-1 – concentrated Na+X− solution” systems, where X = Cl−, Br−, I−, NO2−, NO3−, ClO4− and CrO42−. In the case of NaNO2, NaClO4, Na2CrO4, and NaI a combination of bumper and shock-absorber behaviors with a partial irreversible solution intrusion was observed, whereas a fully reversible spring behavior is demonstrated for the intrusion–extrusion of NaBr, NaCl and NaNO3 solutions. In comparison with water, the intrusion pressure increases for all the solutions except for NaClO4 one. The irreversibility of intrusion decreases with a dilution rate, and the behavior of the corresponding systems with diluted solutions becomes very close. The variation of the system behavior and intrusion pressure values can be related to a different affinity of the corresponding anions for the pores of Silicalite-1. The samples before and after intrusion–extrusion experiments were characterized using several structural and physicochemical methods (XRD, TGA, solid-state NMR, and N2 physisorption), but no significant structural difference was observed.


 Please wait while we load your content...
Please wait while we load your content...