Photoinduced ICT vs. excited rotamer intercoversion in two quadrupolar polyaromatic N-methylpyridinium cations†
Abstract
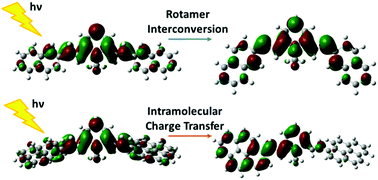
The excited state dynamics of two quadrupolar polyaromatic N-methylpyridinium cations have been fully investigated in order to acquire detailed information on their photo-induced behavior. The two molecules are symmetric push–pull compounds having a D–π–A+–π–D motif, with the same electron-acceptor central unit (A = N-methylpyridinium) and two distinctive electron-donor polyaromatic side groups (D), namely naphthyl and pyrenyl substituents. Both molecules undergo charge transfer during the absorption, as revealed by a significant solvatochromism exhibited with solvent polarity, but the fate of their excited state was found to be markedly different. The careful analysis of the data gathered from femtosecond-resolved fluorescence up-conversion and transient absorption experiments, supported by DFT quantum mechanical calculations and temperature-dependent stationary measurements, shows the leading role of intramolecular charge transfer, assisted by symmetry breaking, in the pyrenyl derivative and that of rotamer interconversion in the naphthtyl one. Both excited state processes are controlled by the viscosity rather than polarity of the solvent, and they occur during inertial solvation in low-viscous media and lengthening up to tens of picoseconds in highly viscous solvents.


 Please wait while we load your content...
Please wait while we load your content...