CO ligands stabilize metal chalcogenide Co6Se8(CO)n clusters via demagnetization†
Abstract
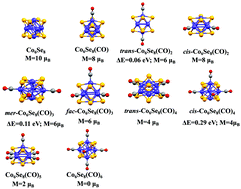
The role of carbon monoxide ligands on the magnetic moment of Co6Se8(CO)n clusters, n = 0–6 was investigated to better understand the interplay between the electronic structure of metal chalcogenide clusters and their ligands. We find that the addition of CO ligands to Co6Se8 results in the gradual demagnetization of the cluster. Generally, the addition of a CO ligand effectively adds two electrons to the cluster that occupy deeper states and further pushes up an antibonding orbital out of the valence manifold of cluster states. Through such processes, the fully ligated Co6Se8(CO)6 cluster attains a closed electronic shell with a large gap between occupied and unoccupied states. Each removal of a CO ligand from the cluster then stabilizes an antibonding state that adds unoccupied states to the valence manifold. Such a cluster with partially filled states may either deform as in a Jahn–Teller distortion to quench the spin, or maintain its core structure and be stabilized in a high spin state as in Hund's rules. As these clusters generally maintain their octahedral core, the high spin state prevails and the removal of the ligands results in an increase in spin multiplicity.


 Please wait while we load your content...
Please wait while we load your content...