Photoelectron spectroscopy and density functional theory studies of (fructose + (H2O)n)− (n = 1–5) anionic clusters†
Abstract
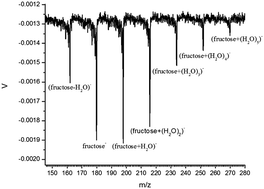
Photoelectron spectroscopy (PES) and density functional theory (DFT) based calculations are executed to characterize gas phase, isolated (fructose + (H2O)n)− (n = 1–5) anionic species produced using a matrix assisted laser desorption ionization (MALDI) method. Gas phase, isolated (fructose + (H2O)n)− (n = 1–5) cluster anions mainly exist as open chain structures with conformational and positional isomers in the present experiments. Some cyclic structures of (fructose + (H2O)n)− (n = 3, 4) are apparently present in the experiments and their VDEs can contribute to the lower energy shoulders of PES features observed for (fructose + (H2O)n)− (n = 3, 4). Cyclic (fructose + (H2O)n)− (n = 1–5) clusters have the added electron as dipole bound, whereas open chain structures have the added electron in a valence orbital. Water molecules in open chain anions predominantly interact with the (1)C side (including (1)OH, (2)O, and (3)OH) of fructose−: they finally form a quasi-cubic structure with OH groups and carbonyl O in the most stable structures for (fructose + (H2O)4)− and (fructose + (H2O)5)− cluster anions. Water molecules solvating cyclic anions form water–water hydrogen bond networks that preferentially interact with OH groups at the (1), (2), and (3) positions of fructose pyranose anions, and the (3), (4), and (6) positions of fructose furanose anions. Structures of neutral (fructose + (H2O)n) (n = 1–5) have pyranose structures as the lower energy isomers rather than open chain structures: this observation is consistent with the fructose solution tautomeric equilibrium with neutral fructose pyranose being the preponderant species. Water molecules also tend to form water–water hydrogen bond networks, interacting with OH groups at (1), (2), and (3) positions for neutral pyranose conformations.


 Please wait while we load your content...
Please wait while we load your content...