Ionic liquids with anions based on fluorosulfonyl derivatives: from asymmetrical substitutions to a consistent force field model†
Abstract
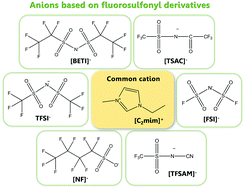
Herein, seven anions including four imide-based, namely bis[(trifluoromethyl)sulfonyl]imide (TFSI), bis(fluorosulfonyl)imide (FSI), bis[(pentafluoroethyl)sulfonyl]imide (BETI), 2,2,2-trifluoromethylsulfonyl-N-cyanoamide (TFSAM) and 2,2,2-trifluoro-N-(trifluoromethylsulfonyl) acetamide (TSAC), and two sulfonate anions, trifluoromethanesulfonate (triflate, TF) and nonafluorobutanesulfonate (NF), are considered and compared. The volumetric mass density and dynamic viscosity of five ionic liquids containing these anions combined with the commonly used 1-ethyl-3-methylimidazolium cation (C2C1im), [C2C1im][FSI], [C2C1im][BETI], [C2C1im][TFSAM], [C2C1im][TSAC] and [C2C1im][NF] are measured in the temperature range of 293.15 ≤ T/K ≤ 353.15 and at atmospheric pressure. The results show that [C2mim][FSI] and [C2mim][TFSAM] exhibit the lowest densities and viscosities among all the studied ionic liquids. The experimental volumetric data is used to validate a more consistent re-parameterization of the CL&P force field for use in MD simulations of ionic liquids containing the ubiquitous bis[(trifluoromethyl)sulfonyl]imide and trifluoromethanesulfonate anions and to extend the application of the model to other molten salts with similar ions.


 Please wait while we load your content...
Please wait while we load your content...