Photodissociation of the CH3O and CH3S radical molecules: an ab initio electronic structure study
Abstract
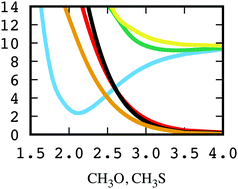
The electronic states and the spin–orbit couplings between them involved in the photodissociation process of the radical molecules CH3X, CH3X → CH3 + X (X = O, S), taking place after the Ã(2A1) ← ![[X with combining tilde]](https://www.rsc.org/images/entities/char_0058_0303.gif) (2E) transition, have been investigated using highly correlated ab initio techniques. A two-dimensional representation of both the potential-energy surfaces (PESs) and the couplings is generated. This description includes the C–X dissociative mode and the CH3 umbrella mode. Spin–orbit effects are found to play a relevant role in the shape of the excited state potential-energy surfaces, particularly in the CH3S case where the spin–orbit couplings are more than twice more intense than in CH3O. The potential surfaces and couplings reported here for the present set of electronic states allow for the first complete description of the above photodissociation process. The different photodissociation mechanisms are analyzed and discussed in light of the results obtained.
(2E) transition, have been investigated using highly correlated ab initio techniques. A two-dimensional representation of both the potential-energy surfaces (PESs) and the couplings is generated. This description includes the C–X dissociative mode and the CH3 umbrella mode. Spin–orbit effects are found to play a relevant role in the shape of the excited state potential-energy surfaces, particularly in the CH3S case where the spin–orbit couplings are more than twice more intense than in CH3O. The potential surfaces and couplings reported here for the present set of electronic states allow for the first complete description of the above photodissociation process. The different photodissociation mechanisms are analyzed and discussed in light of the results obtained.


 Please wait while we load your content...
Please wait while we load your content...