Aggregation-induced visible light absorption makes reactant 1,2-diisocyanoarenes act as photosensitizers in double radical isocyanide insertions†
Abstract
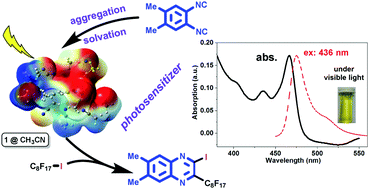
The joint computational and experimental efforts reveal that the organic molecule 1,2-diisocyano-4,5-dimethylbenzene (1) acts as both a reactant and a photosensitizer (PS) in a metal-free reaction with perfluoroalkylhalide (2) to produce 2-perfluoroalkyl quinoxalines (3) under visible light. Both the π–π stacking aggregation in crystals and the solvation in various solvents of PS 1 exhibited visible-light absorption at 466 nm in spite of its smaller coefficient than that of the ultraviolet-light absorption. Such an aggregation-assisted visible-light absorption phenomenon is rationalized by theoretical calculations of the condensed-phase properties with the consideration of the explicit polarization effect from the neighboring molecules. Upon irradiation with different wavelengths, the emission colors changed from navy to bright yellow. Fluorescence lifetime measurements show that the emission of 1 comes from its singlet excited state. The aggregation induced emission when excited at 420 nm has a shorter lifetime (0.45 ns) than that of the emission from isolated molecules (2.71 ns) when excited at 381 nm. It is conceived that the aggregation assisted visible light absorption properties may be general in other photo-reactive molecules, such as 1,4-diisocyano-2,5-dimethylbenzene (4), 1,4-dicyanobenzene (5), and 1,4-diisocyanobenzene (6), which are widely used in many photochemical reactions in the absence of any external photosensitizer.


 Please wait while we load your content...
Please wait while we load your content...