Theoretical design and mechanistic study of the metal-free reduction of CO2 to CO†
Abstract
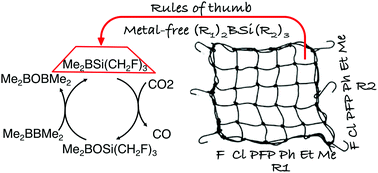
A strategy for the reduction of CO2 to CO by or catalyzed by metal-free silylboranes has been proposed with the aid of density functional theory (DFT) computations. We showed that one oxygen atom of CO2 can be abstracted by silylboranes without catalysts or by diboranes in the presence of silylborane catalysts with surprisingly low free-energy barriers so that the reaction can be realized under mild experimental conditions. To achieve this, the reduction mechanism of CO2 by a hierarchy of silylboranes (R1)2BSi(R2)3 was systematically investigated. Several rules of thumb were obtained to guide the design of silylboranes with high activity toward CO2 reduction. After considering many factors, such as side reactions, the stability of the silylboranes, and the solvent effect, two silylboranes, (PFP)2BSi(CH2F)3 and Me2BSi(CH2F)3, suitable for the reduction of CO2 under mild experimental conditions were designed. The overall free-energy barriers for the reduction of CO2 by the two silylboranes are just 26.1–27.0 kcal mol−1 and 28.1–28.9 kcal mol−1, respectively, at 298.15 K in solution. We further showed that CO2 can be reduced to CO by diborane Me2BBMe2 using Me2BSi(CH2F)3 as the catalyst. The overall free-energy barrier for this catalytic reaction is just 30.6–30.7 kcal mol−1 at 298.15 K in solution.


 Please wait while we load your content...
Please wait while we load your content...