Rotational spectroscopy of the methyl glycidate–water complex: conformation and water and methyl rotor tunnelling motions†
Abstract
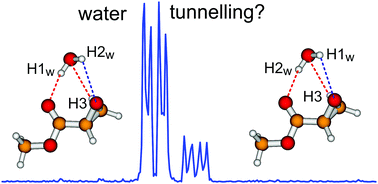
Methyl glycidate is a chiral epoxy ester whose structure and characteristic functional groups can be used to model biological events involving much larger chiral esters on the molecular scale. Since biological molecules function in aqueous solution, it is of interest to obtain detailed knowledge of the initial few steps of solvation using high resolution spectroscopy. In the current study, rotational spectra of methyl glycidate monohydrate were investigated by using a chirped-pulse and a cavity-based Fourier transform microwave spectrometer. Quantum theory of atoms in molecules and non-covalent interaction analyses were performed for the observed monohydrate to characterize non-covalent interactions in the system. In the observed monohydrate, methyl glycidate takes on its most stable monomeric form, while water serves as a hydrogen bond donor to the carbonyl group and as a hydrogen bond acceptor to the hydrogen atom of the CH2 group on the epoxide ring, and additionally forms a close contact to the epoxide oxygen atom. Unexpectedly, water tunnelling splittings on the order of tens to hundreds of kHz were detected. A water tunnelling path was proposed and a surprisingly low tunnelling barrier of about 2 kJ mol−1 was calculated. The proposed tunnelling path is asymmetric in the forward and backward tunnelling directions, demonstrating the complicated dynamics of water motions in the system. In addition, splittings due to the methyl internal rotation were observed and analyzed.


 Please wait while we load your content...
Please wait while we load your content...