Optical and magnetic properties of antiaromatic porphyrinoids†
Abstract
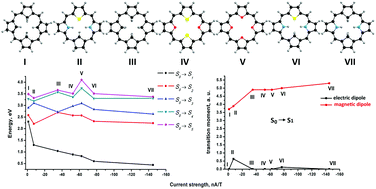
Magnetic and spectroscopic properties of a number of formally antiaromatic carbaporphyrins, carbathiaporphyrins and isophlorins with 4n π electrons have been investigated at density functional theory and ab initio levels of theory. The calculations show that the paratropic contribution to the magnetically induced ring-current strength susceptibility and the magnetic dipole-transition moment between the ground and the lowest excited state are related. The vertical excitation energy (VEE) of the first excited state decreases with increasing ring-current strength susceptibility, whereas the VEE of the studied higher-lying excited states are almost independent of the size of the ring-current strength susceptibility. Strong antiaromatic porphyrinoids, based on the magnitude of the paratropic ring-current strength susceptibility, have small energy gaps between the highest occupied and lowest unoccupied molecular orbitals and a small VEE of the first excited state. The calculations show that only the lowest S0 → S1 transition contributes signficantly to the magnetically induced ring-current strength susceptibility of the antiaromatic porphyrinoids. The decreasing optical gap combined with a large angular momentum contribution to the magnetic transition moment from the first excited state explains why molecules III–VII are antiaromatic with very strong paratropic ring-current strength susceptibilities. The S0 → S1 transition is a magnetic dipole-allowed electronic transition that is typical for antiaromatic porphyrinoids with 4n π electrons.


 Please wait while we load your content...
Please wait while we load your content...