Thermal evolution of MnxOy nanofibres as catalysts for the oxygen reduction reaction
Abstract
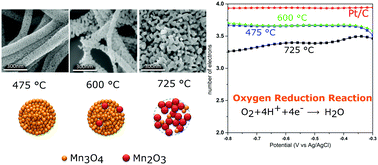
Manganese oxides (MnxOy) are considered as a promising catalyst alternative to platinum in fuel cell applications. In fact, a proper catalyst is needed in order to facilitate the oxygen reduction reaction (ORR) at the cathode, and platinum is considered the best material due to its low overpotential for this reaction. Contrary to platinum, MnxOy is inexpensive, environmentally friendly and can be shaped into several nanostructures; furthermore, most of them show significant electro-catalytic performance. Several strategies have been carried out in order to increase their efficiency, by preparing light and high-surface area materials. In this framework, nanofibres are among the most promising nanostructures that can be used for this purpose. In this work, a study of the thermal, morphological and catalytic behavior of MnxOy nanofibres obtained through the electrospinning technique is proposed. Emphasis is given to the thermal evolution of the precursors, proposing a possible crystallization mechanism of the different manganese oxides obtained. It turns out that manganese oxide nanofibres exhibit good catalytic performance for the ORR, comparable to those obtained by using Pt-based catalysts.


 Please wait while we load your content...
Please wait while we load your content...