Double-ring tubular (B2O2)n clusters (n = 6–42) rolled up from the most stable BO double-chain ribbon in boron monoxides†
Abstract
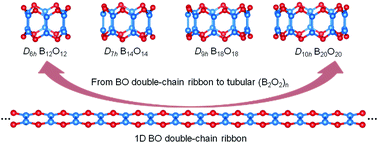
Based on extensive global searches and first-principles theory calculations, we present herein the possibility of double-ring tubular (B2O2)n clusters (n = 6–42) (2–10) rolled up from the most stable one-dimensional (1D) BO double-chain ribbon (1) in boron monoxides. Tubular (3D) (B2O2)n clusters (n ≥ 6) are found to be systematically much more stable than their previously proposed planar (2D) counterparts, with a 2D–3D structural transition at B12O12 (2). Detailed bonding analyses on 3D (B2O2)n clusters (2–10) and their precursor 1D BO double-chain ribbon (1) reveal two delocalized B–O–B 3c-2e π bonds over each edge-sharing B4O2 hexagonal unit which form a unique 6c-4e o-bond to help stabilize the systems. The IR, Raman, UV-vis, and photoelectron spectra of the concerned species are computationally simulated to facilitate their experimental characterization.


 Please wait while we load your content...
Please wait while we load your content...