The ionic versus metallic nature of 2D electrides: a density-functional description
Abstract
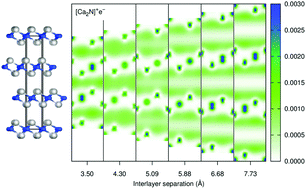
The two-dimensional (2D) electrides are a highly unusual class of materials, possessing interstitial electron layers sandwiched between cationic atomic layers of the solid. In this work, density-functional theory, with the exchange-hole dipole moment dispersion correction, is used to investigate exfoliation and interlayer sliding of the only two experimentally known 2D electrides: [Ca2N]+e− and [Y2C]2+(2e−). Examination of the valence states during exfoliation identifies intercalated electrons in the bulk and weakly-bound surface-states in the fully-expanded case. The calculated exfoliation energies for the 2D electrides are found to be much higher than for typical 2D materials, which is attributed to the ionic nature of the electrides and the strong Coulomb forces governing the interlayer interactions. Conversely, the calculated sliding barriers are found to be quite low, comparable to those for typical 2D materials, and are effectively unchanged by exclusion of dispersion. We conjecture that the metallic nature of the interstitial electrons allows the atomic layers to move relative to each other without significantly altering the interlayer binding. Finally, comparison with previous works reveals the importance of a system-dependent dispersion correction in the density-functional treatment.


 Please wait while we load your content...
Please wait while we load your content...