Photoelectron spectroscopy of isolated luciferin and infraluciferin anions in vacuo: competing photodetachment, photofragmentation and internal conversion†
Abstract
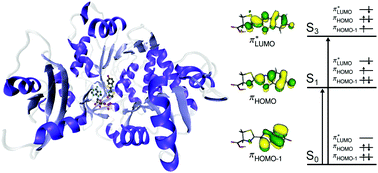
The electronic structure and excited-state dynamics of the ubiquitous bioluminescent probe luciferin and its furthest red-shifted analogue infraluciferin have been investigated using photoelectron spectroscopy and quantum chemistry calculations. In our electrospray ionization source, the deprotonated anions are formed predominantly in their phenolate forms and are directly relevant to studies of luciferin and infraluciferin as models for their unstable oxyluciferin and oxyinfraluciferin emitters. Following photoexcitation in the range 357–230 nm, we find that internal conversion from high-lying excited states to the S1(1ππ*) state competes efficiently with electron detachment. In infraluciferin, we find that decarboxylation also competes with direct electron detachment and internal conversion. This detailed spectroscopic and computational study defines the electronic structure and electronic relaxation processes of luciferin and infraluciferin and will inform the design of new bioluminescent systems and applications.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...