Use of NH (A3Π–X3Σ−) sonoluminescence for diagnostics of nonequilibrium plasma produced by multibubble cavitation†
Abstract
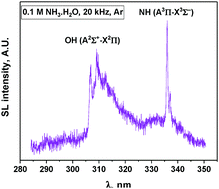
In this work, the sonoluminescence of NH radicals has been evaluated as a new spectroscopic probe for the nonequilibrium plasma produced by multibubble cavitation in liquids. The experiments were performed in aqueous ammonia solutions subjected to power ultrasound at low and high frequencies and under two different rare gases (Ar and Xe). Sonoluminescence (SL) spectroscopy focuses on the emission of the two present systems: NH (A3Π–X3Σ−) and OH (A2Σ+–X2Π). Both spectroscopic systems indicate the absence of thermal equilibrium during bubble collapse (Tv > Tr) irrespective of the saturating gas. When Ar is used as the saturating gas, these emissions can be fitted using Specair software and the corresponding rovibronic temperatures are derived. Both species indicate a net increase in vibrational temperatures with the US frequency. In Xe, the SL spectra exhibit OH (C2Σ+–A2Σ+) and NH (c1Π–a1Δ) emission bands indicating a higher electron temperature compared to Ar. However, in Xe, the SL spectra cannot be satisfactorily fitted because of significant line broadening. The estimation of the intrabubble pressure via SL simulation using Specair software is discussed. Monitoring of the sonochemical activity indicates the formation of H2 and N2H4, while no H2O2 accumulates under these conditions. In the presence of Xe, NO is also formed as a sonolysis product. The appearance of new possible reaction pathways under Xe is made possible by the higher plasma electron density and correlates with SL data.


 Please wait while we load your content...
Please wait while we load your content...