Water dynamics in hydrated amorphous materials: a molecular dynamics study of the effects of dehydration in amorphous calcium carbonate
Abstract
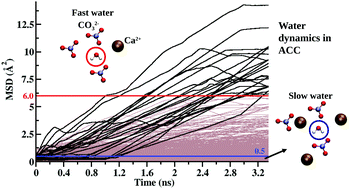
Amorphous calcium carbonate (ACC) is often a critical transient phase in the formation of crystalline phases of CaCO3via dehydration of hydrated ACC. The behavior of the water molecules plays a pivotal role in this transformation. We report here the dynamics of water molecules in ACC at hydration levels from CaCO3·1H2O to CaCO3·0.25H2O using molecular dynamics (MD) simulations. Due to the presence of highly hydrophilic Ca2+ and the strong H-bond acceptor CO32−, most of the water molecules in our simulations have restricted translational and orientational dynamics. These are referred here as ‘slow water’. However, a small fraction of them show high diffusivity at all hydration states and are referred here as ‘fast water’. The computed diffusion coefficients of the slow waters, as extrapolated from simulation results, yield diffusion distances of the order of μm on the 1 hour time scale, consistent with rapid dehydration of ACC nano-particles in the laboratory. We correlate our fast waters with the water molecules in the percolating water-rich, Ca2+-deficient nano-pores in the structure of Goodwin et al. [Chem. Mater., 2010, 22, 3197], obtained by analysis of X-ray scattering data, and our slow waters with those in the Ca2+-rich volumes with less water in their model. The fast waters can be considered to be free rotors on the ns time scale and have orders of magnitude shorter rotational relaxation times than the slow waters.


 Please wait while we load your content...
Please wait while we load your content...