Hydrophobic fluorine mediated switching of the hydrogen bonding site as well as orientation of water molecules in the aqueous mixture of monofluoroethanol: IR, molecular dynamics and quantum chemical studies†
Abstract
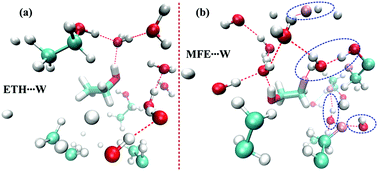
The local structures between water–water, alcohol–water and alcohol–alcohol have been investigated for aqueous mixtures of ethanol (ETH) and monofluoroethanol (MFE) by the deconvolution of IR bands in the OH stretching region, molecular dynamics simulation and quantum chemical calculations. It has been found that the addition of a small amount of ETH into the aqueous medium increases the strength of the hydrogen bonds between water molecules. In an aqueous mixture of MFE, the substitution of a single fluorine induces a change in the orientation as well as the hydrogen bonding site of water molecules from the oxygen to the fluorine terminal of MFE. The switching of the hydrogen bonding site of water in the aqueous mixture of MFE results in comparatively strong hydrogen bonds between MFE and water molecules as well as less clustering of water molecules, unlike the case of the aqueous mixture of ETH. These findings about the modification of a hydrogen bond network by the hydrophobic fluorine group probably make fluorinated molecules useful for pharmaceutical as well as biological applications.


 Please wait while we load your content...
Please wait while we load your content...