First principles study of the Mn-doping effect on the physical and chemical properties of mullite-family Al2SiO5†
Abstract
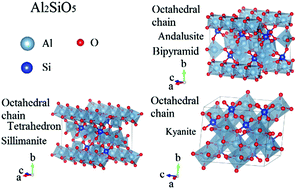
Transition metal (TM) modification is a common strategy for converting an earth-abundant mineral into a cost-effective catalyst for industrial applications. Among a variety of minerals, Al2SiO5, which has three phases, andalusite, sillimanite and kyanite, is emerging as a promising candidate for new catalyst development. In this paper, we use Mn to demonstrate the rationale of 3d TM doping at the Al sites in each of these three phases through first-principles calculations and the cluster expansion method. The results of cluster expansion show that Mn has a strong site preference for the six-coordinated Al octahedral chains in the andalusite and sillimanite phases, while distributing randomly in the kyanite phase. Moreover, Mn can only replace Al in sillimanite and kyanite in low concentrations; however, higher concentrations of Mn can replace Al in andalusite. We found that the concentration sensitivity is due to the Jahn–Teller distortion and 3d orbital splitting. This finding can also explain the low doping concentrations of other 3d TMs (Fe, Cr and V) in Al2SiO5 compounds. Based on the calculated Helmholtz free energy, we constructed a (MnxAl1−x)AlSiO5 temperature-composite phase diagram, which explains the physical mechanisms behind the results for 3d transition metal doping and phase transitions in Al2SiO5. This work could shed light on the related physics, chemistry, and geoscience of (MnxAl1−x)AlSiO5, and more importantly, a design rationale for the engineering of cheap catalysts.


 Please wait while we load your content...
Please wait while we load your content...