Confined phase separation of aqueous–organic nanodroplets†
Abstract
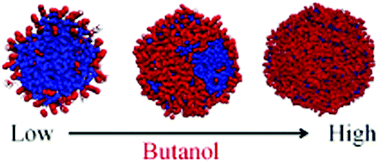
Nano-confined supercooled water occurs frequently in aqueous–organic aerosol nanodroplets that are ubiquitous in the atmosphere and in many industrial processes such as natural gas refining. The structure of these nanodroplets is important because it influences droplet growth and evaporation rates, nucleation rates, and radiative properties. We used classical molecular dynamics (MD) simulations to study the structures of binary water–butanol nanodroplets for several temperatures and droplet sizes. Water–butanol cross interactions are calculated using a Lennard-Jones (LJ) potential with non-bonded specific parameters adjusted to reproduce the experimentally observed mutual solubilities of water–butanol at 295 K. To compare with the results of the density functional theory (DFT) of aqueous-organic nanodroplets [Phys. Chem. Chem. Phys., 2006, 8, 1266–1270], we focus on T = 250 K. Our simulations show three different nanodroplet structures depending on the butanol concentration. For low concentrations, we observe a core–shell (CS) structure in which a butanol shell completely wets a water-rich core. For high concentrations, a well-mixed (WM) structure occurs as the water and the butanol become fully miscible. For intermediate concentrations of butanol, we find a distinct phase-separated Russian Doll–Shell (RDS) structure. This RDS structure consists of a roughly ellipsoidal water-rich droplet partially wetted by a well-mixed water/butanol convex lens (RD) and this lens-on-sphere structure is coated by a thin shell of butanol. We also examined the stability of our RDS structure at a higher temperature and found that at 295 K, the RDS structure had transformed into a well-mixed droplet, presumably due to the increase in the mutual solubility of water and butanol. Finally, we performed calculations using classical density functional theory for conditions that should favor the RDS structure. The results closely resembled those found using MD.


 Please wait while we load your content...
Please wait while we load your content...