A topological study of chemical bonds under pressure: solid hydrogen as a model case†
Abstract
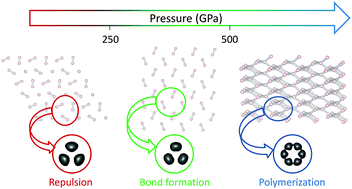
It is now well recognized that a fundamental understanding of the rules that govern chemistry under pressure is still lacking. Hydrogen being the “simplest” element as well as a central core to high pressure physics, we undertake a general study of the changes in the chemical bonding under pressure. We start from a simple trimer unit that has been found in high pressure phases, whose behavior has been found to reveal the basics of hydrogen polymerization under pressure. Making use of bond analysis tools, mainly the NCI (noncovalent interactions) index, we show that polymerization takes place in three steps: dipolar attraction, repulsion and bond formation. The use of a 1D Wigner–Seitz radius allowed us to extend the conclusions to 3D networks and to analyze their degree of polymerization. On the one hand, this approach provides new insight into the polymerization of hydrogen. On the other hand, it shows that complicated molecular solids can be understood from cluster models, where correlated methods can be applied, main differences in solid state arising at the transition points, where breaking/forming of bonds happens at once instead of continuously like in the cluster model.


 Please wait while we load your content...
Please wait while we load your content...