Towards completing the cyclopropenylidene cycle: rovibrational analysis of cyclic N3+, CNN, HCNN+, and CNC−
Abstract
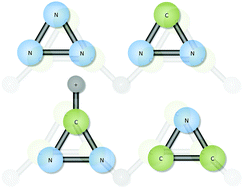
The simple aromatic hydrocarbon, cyclopropenylidene (c-C3H2), is a known, naturally-occurring molecule. The question remains as to whether its isoelectronic, cyclic, fellow aromatics of c-N3+, c-CNN, HCNN+, and c-CNC− are as well. Each of these are exciting objects for observation of Titan, and the rotational constants and vibrational frequencies produced here will allow for remote sensing of Titan's atmosphere or other astrophysical or terrestrial sources. None of these four aromatic species are vibrationally strong absorbers/emitters, but the two ions, HCNN+ and c-CNC−, have dipole moments of greater than 3 D and 1 D, respectively, making them good targets for rotational spectroscopic observation. Each of these molecules is shown here to exhibit its own, unique vibrational properties, but the general trends put the vibrational behavior for corresponding fundamental modes within close ranges of one another, even producing nearly the same heavy atom, symmetric stretching frequencies for HCNN+ and c-C3H2 at 1600 cm−1. The c-N3+ cation is confirmed to be fairly unstable and has almost no intensity in its ν2 fundamental. Hence, it will likely remain difficult to characterize experimentally.


 Please wait while we load your content...
Please wait while we load your content...