Separate mechanisms of ion oligomerization tune the physicochemical properties of n-butylammonium acetate: cation-base clusters vs. anion-acid dimers†
Abstract
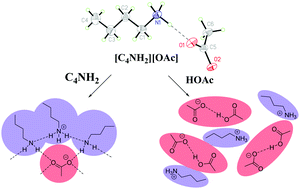
We investigated the ability of the ions comprising protic ionic liquids to strongly interact with their neutral acid and base forms through the characterization of n-butylammonium acetate ([C4NH3][OAc]) in the presence of excess n-butylamine (C4NH2) or excess acetic acid (HOAc). The conjugate and parent acid or base form new nonstoichiometric, noncovalently bound species (i.e., oligomeric ions) which change the physical and chemical properties of the resulting liquids, thus offering tunability. The effects of adding C4NH2 or HOAc to [C4NH3][OAc] on the resulting thermal and spectroscopic properties differ and suggest that C4NH2 interacts primarily with [C4NH3]+ to form 3-dimensional polymeric networks likely similar to those in H2O/[H3O]+, while HOAc interacts primarily with [OAc]− to form oligomeric ions (e.g., [H(OAc)2]−). The densities of the systems increased with the increase of acid content and reached a maximum when the acid molar fraction was 0.90, but decreased with increasing amine concentration. The viscosities decreased significantly with increasing acid or base concentration. The solvent properties of the mixtures were assessed by measuring the solubilities of benzene, ethyl acetate, diethyl ether, heptane, ibuprofen free acid, and lidocaine free base. The solubilities of the organic solutes and active pharmaceutical ingredients can be tuned with the concentration of acid or amine in the mixtures. In addition, crystallization of the active pharmaceutical ingredients can be induced with the modification of the composition of the mixtures. These observations support the usage of these mixtures for the synthesis and purification of acid or basic active pharmaceutical ingredients in the pharmaceutical industry.


 Please wait while we load your content...
Please wait while we load your content...