Fluorination-enriched electronic and magnetic properties in graphene nanoribbons
Abstract
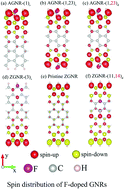
The feature-rich electronic and magnetic properties of fluorine-doped graphene nanoribbons are investigated by the first-principles calculations. They arise from the cooperative or competitive relations among the significant chemical bonds, finite-size quantum confinement and edge structure. There exist C–C, C–F, and F–F bonds with multi-orbital hybridizations. Fluorine adatoms can create p-type metals or concentration- and distribution-dependent semiconductors, depending on whether the π bonding is seriously suppressed by the top-site chemical bonding. Furthermore, five kinds of spin-dependent electronic and magnetic properties cover the non-magnetic and ferromagnetic metals, non-magnetic semiconductors, and anti-ferromagnetic semiconductors with/without spin splitting. The diverse essential properties are clearly revealed in the spatial charge distribution, spin density, and orbital-projected density of states.


 Please wait while we load your content...
Please wait while we load your content...