Silica nanoparticle monolayers on a macroion modified surface: formation mechanism and stability
Abstract
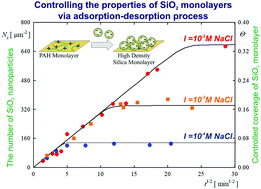
Even though silica nanoparticles and their monolayers find a broad field of applications, only a few studies providing a quantitative description of silica nanoparticle deposition at solid/liquid interfaces have been reported in the literature. Given the deficit of reliable experimental data, the goal of this work is to acquire thorough physicochemical characteristics of amorphous silica nanoparticle deposition. Silica nanoparticle monolayers of controlled coverage were formed on macroion (PAH)-modified mica. The size of the particles determined by dynamic light scattering (DLS), atomic force microscopy (AFM) and scanning electron microscopy (SEM) was equal to 28 nm. The electrophoretic mobility and the zeta potential of the particles were also determined as a function of ionic strength and pH. Using a well-defined suspension, systematic studies of particle deposition kinetics were carried out. The coverage of the self-assembled particle monolayers was determined by AFM and SEM imaging. Particle deposition was carried out under diffusion controlled transport conditions. For long deposition times, the saturation coverage was attained, systematically increasing with ionic strength up to 0.48 for I = 0.15 M NaCl. The deposition kinetic runs were adequately interpreted using the random sequential adsorption (RSA) model. This model was also used to determine the specific density of silica particles that confirmed their porous structure. In addition, the particle desorption kinetics was studied using AFM and SEM methods. It was confirmed that silica nanoparticle desorption was negligible within the time period of 60 hours. Our experimental data proved, therefore, that it is feasible to produce uniform and stable silica particle monolayers of desired coverage in the self-assembly processes, controlled by the bulk suspension concentration and the ionic strength. Such monolayers may find practical applications as substrates for selective protein and nanoparticle deposition, or various catalytic applications.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...