Interaction of FeO− with water: anion photoelectron spectroscopy and theoretical calculations†
Abstract
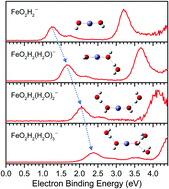
The interactions of FeO− with water molecules were studied by using photoelectron spectroscopy and density functional theoretical calculations. It is found that a dihydroxyl species, Fe(OH)2−/0, can be formed when FeO−/0 interacts with the first water molecule. The complexes formed via the interactions between FeO−/0 and n water molecules can be viewed as Fe(OH)2(H2O)n−1−/0, in which (n − 1)H2O molecules interact with a Fe(OH)2 core. For Fe(OH)2−/0 and Fe(OH)2(H2O)−, the Fe(OH)2 unit has two conformers with the two OH groups oriented differently. The vertical detachment energies (VDEs) of FeO2H2(H2O)n−1− (n = 1–4) are measured to be 1.25 ± 0.04, 1.66 ± 0.04, 2.06 ± 0.04, and 2.37 ± 0.04 eV, respectively, by experiment. It is also worth mentioning that in the FeO2H2(H2O)− anion the water molecule interacts with the Fe(OH)2 core by forming a hydrogen bond with one of the OH groups, while in neutral FeO2H2(H2O), the water molecule interacts with the Fe atom of the Fe(OH)2 core via its O atom.


 Please wait while we load your content...
Please wait while we load your content...