DFT study of CO2 conversion on InZr3(110) surface†
Abstract
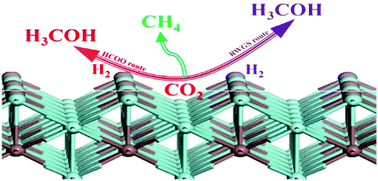
Methanol and methane synthesis from CO2 hydrogenation on a InZr3(110) surface has been studied using density functional theory calculations. The CO2 can be chemically adsorbed via a polydentated configuration and the H2 molecule can dissociate to H atoms spontaneously. The methanol is primarily formed via the HCOO route instead of the RWGS route, due to its higher activation barrier of 1.35 eV for HCO hydrogenation. In the HCOO route, the adsorbed CO2 consecutively hydrogenates to form HCOO, H2COO and the H3CO species. The H3COH is produced via the reaction of H3CO with a surface OH group. Furthermore, the C–O bonds of CO, CHO, CH2O and CH3O species prefer to dissociate to C, CH, CH2 CH3 and surface O species. Methane is formed via the hydrogenation of CHx (x = 0–3) monomers with the highest activation barrier of 1.19 eV for CH3 hydrogenation, which is higher than that of the hydrogenation of H2COO in methanol synthesis via the HCOO route. The surface O species formed during CO2 hydrogenation reacts with the adsorbed H2 molecule to produce an OH group which reacts with a surface H atom to form H2O with an activation barrier of 1.13 eV, which then desorbs to the gas phase. Our calculated results indicate that the InZr3 alloy is a potential candidate catalyst for CO2 utilization and conversion.


 Please wait while we load your content...
Please wait while we load your content...