Proteins at air–water and oil–water interfaces in an all-atom model†
Abstract
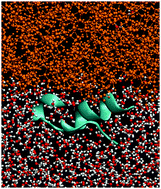
We study the behavior of five proteins at the air–water and oil–water interfaces by all-atom molecular dynamics. The proteins are found to get distorted when pinned to the interface. This behavior is consistent with the phenomenological way of introducing the interfaces in a coarse-grained model through a force that depends on the hydropathy indices of the residues. Proteins couple to the oil–water interface stronger than to the air–water one. They diffuse slower at the oil–water interface but do not depin from it, whereas depinning events are observed at the other interface. The reduction of the disulfide bonds slows the diffusion down.


 Please wait while we load your content...
Please wait while we load your content...