Prediction of hypervalent molecules: investigation on MnC (M = Li, Na, K, Rb and Cs; n = 1–8) clusters†
Abstract
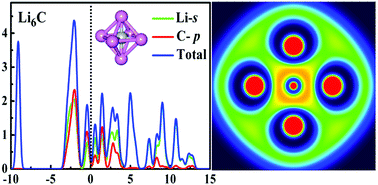
New hypervalent molecules have emerged from a systematic exploration of the structure and bonding of MnC (M = Li, Na, K, Rb and Cs; n = 1–8) clusters via an unbiased CALYPSO structure investigation combined with density functional theory. The global minimum structures are obtained at the B3LYP/6-311+G* and CCSD(T)/6-311+G* levels of theory. The observed growth behavior clearly indicates that the ground state of MnC (M = Li, Na, K, Rb and Cs; n = 1–8) is transformed from a planar to a three-dimensional (3D) structure at n = 4. A maximum of six alkali atoms can be bound atomically to a carbon atom. The determination of the averaged binding energies Eb(n), fragmentation energies ΔE(n) and HOMO–LUMO energy gaps unambiguously supports the stability of M6C. This indicated conclusively that 6 is a magic Li-coordination number for C. The nature of bonding is further investigated by an insightful analysis of the highest occupied molecular orbital (HOMO) and the topology of chemical bonds for the most stable clusters. In the final step, electron localization functions (ELF) and density of states (DOS) are determined in order to consolidate the acquired information on the studied electronic structures.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...