Neutron vibrational spectroscopic studies of novel tire-derived carbon materials†
Abstract
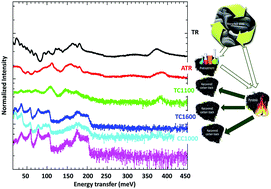
Sulfonated tire-derived carbons have been demonstrated to be high value-added carbon products of tire recycling in several energy storage system applications including lithium, sodium, potassium ion batteries and supercapacitors. In this communication, we compared different temperature pyrolyzed sulfonated tire-derived carbons with commercial graphite and unmodified/non-functionalized tire-derived carbon by studying the surface chemistry and properties, vibrational spectroscopy of the molecular structure, chemical bonding such as C–H bonding, and intermolecular interactions of the carbon materials. The nitrogen adsorption–desorption studies revealed the tailored micro and meso pore size distribution of the carbon during the sulfonation process. XPS and neutron vibrational spectra showed that the sulfonation of the initial raw tire powders could remove the aliphatic hydrogen containing groups (![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) CH2 and –CH3 groups) and reduce the number of heteroatoms that connect to carbon. The absence of these functional groups could effectively improve the first cycle efficiency of the material in rechargeable batteries. Meanwhile, the introduced –SO3H functional group helped in producing terminal H at the edge of the sp2 bonded graphite-like layers. This study reveals the influence of the sulfonation process on the recovered hard carbon from used tires and provides a pathway to develop and improve advanced energy storage materials.
CH2 and –CH3 groups) and reduce the number of heteroatoms that connect to carbon. The absence of these functional groups could effectively improve the first cycle efficiency of the material in rechargeable batteries. Meanwhile, the introduced –SO3H functional group helped in producing terminal H at the edge of the sp2 bonded graphite-like layers. This study reveals the influence of the sulfonation process on the recovered hard carbon from used tires and provides a pathway to develop and improve advanced energy storage materials.


 Please wait while we load your content...
Please wait while we load your content...