Quantification of nucleobases/gold nanoparticles interactions: energetics of the interactions through apparent binding constants determination†
Abstract
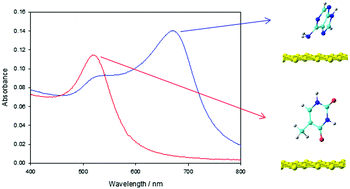
We explore the possibilities of quantifying the interaction of both adenine and thymine with non-functionalized, anionic citrate gold nanoparticles (AuNPs). Following the plasmon absorbance band's red shifts, dependent of the AuNPs aggregation state, apparent binding free energies of the system are obtained for the sum of the two processes (nucleobase interaction plus nanoparticle aggregation). A deconvolution procedure confirms those results. Those apparent binding free energies are, in both cases, in good agreement with previous studies indicating an increased reactivity for adenine. Moreover, density functional theory (DFT) calculations were carried out both to model the structures and to theoretically support and help understand the observed experimental stability of adenine as compared to thymine over gold nanoparticles. Theoretical results indicate the N atom in the amino group of adenine adopts a pyramidal sp3 hybridization character, stabilizing the interaction with Au as compared to thymine.


 Please wait while we load your content...
Please wait while we load your content...