Beryllium-based fluorenes as efficient anion sponges†
Abstract
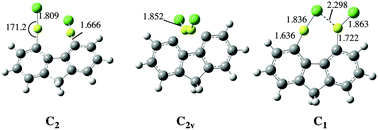
The F−, Cl−, CN−, NO2−, NO3−, and SO42− anion affinities of 4,5-bis(BeX)-fluorene (X = H, F, Cl, CN, NC, and OCH3) derivatives have been calculated at the B3LYP/6-311+G(3df,2p)//B3LYP/6-31+G(d,p) level of theory. The reliability of this approach was assessed using, for some suitable cases, the accurate G4MP2 ab initio composite method as a reference. The values obtained indicate that these derivatives exhibit anion affinities which are among the largest ones reported for single neutral molecules, and therefore these compounds behave as anion sponges, very much as their 1,8-diBeX-naphthalene analogues. This finding seems to confirm that both molecular frameworks, when adequately substituted at positions 1,8 in the case of naphthalene and 4,5 in the case of fluorene, may behave either as proton sponges, when the substituents are good electron donors, such as alkylamino groups, or as anion sponges, when the substituents are BeX groups, which are excellent electron acceptors. The behavior of these compounds in aqueous solution was also investigated. The interaction with water decreases the anion affinities, but still they are very large. More importantly the trends observed do not differ significantly from those found in the gas phase, in particular when monoanions are considered.


 Please wait while we load your content...
Please wait while we load your content...