Anionic fructose-related conformational and positional isomers assigned through PES experiments and DFT calculations†
Abstract
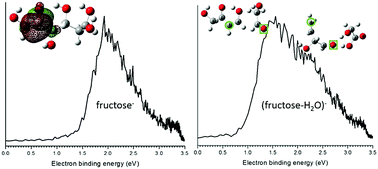
Gas phase, isolated fructose anionic species, fructose−, (fructose-H)−, (fructose-OH)−, and (fructose-H2O)−, are investigated employing anionic photoelectron spectroscopy (PES) combined with density functional theory (DFT) calculations. The PES vertical detachment energies (VDEs) for these anions are determined and, based on these experimental values, their calculated anionic structures are assigned. Generation of these four species through the matrix assisted laser desorption ionization (MALDI) process is sample desorption substrate dependent. The parent anion fructose− exists as a single, dominant open chain structure in the gas phase, with substrate dependent specific conformational isomers. (Fructose-H)− and (fructose-OH)− are mainly produced from the laser ablation process rather than from fragmentation reaction pathways associated with the parent anion species. Both conformational and positional isomers are identified in the gas phase for these latter anions. (Fructose-H2O)− has two types of positional isomers, both of which contribute to two different components of the observed PES feature. The fixed positions for losing an OH group and an H atom, in addition to thermodynamic calculations, provide reaction pathways for generating a dehydration product (open chain structures) from the parent anion (open chain and furanose structures), further demonstrating the active nature of fructose upon capturing an extra electron.


 Please wait while we load your content...
Please wait while we load your content...