Dependence of homogeneous crystal nucleation in water droplets on their radii and its implication for modeling the formation of ice particles in cirrus clouds
Abstract
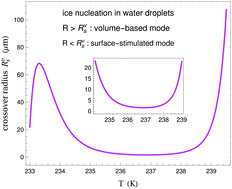
We propose an approximation for the total ice nucleation rate J = J(T,R) in supercooled water droplets as a function of both droplet radius R and temperature T, taking into account both volume-based and surface-stimulated nucleation modes. Its crucial idea is that even in the surface-stimulated mode crystal nuclei initially emerge (as sub-critical clusters) homogeneously in the sub-surface layer, not “pseudo-heterogeneously” at the surface. This mode is negligible in large droplets, but becomes increasingly important with decreasing droplet size and is dominant in small droplets. The crossover droplet radius for the transition of homogeneous ice nucleation from the volume-based mode to the surface-stimulated mode nonmonotonically depends on T from 233 K to 239.5 K, ranging there from ≈2 μm to ≈100 μm. Using experimental data on ice nucleation rates in small droplets, we determine that the ice–air interfacial tension of basal facets of ice crystals monotonically increases from ≈89 dyn cm−1 at T = 234.8 K to ≈91 dyn cm−1 at T = 236.2 K.


 Please wait while we load your content...
Please wait while we load your content...