Mechanisms of carbon monoxide hydrogenation yielding formaldehyde catalyzed by the representative strong mineral acid, H2SO4, and Lewis–Brønsted superacid, HF/AlF3
Abstract
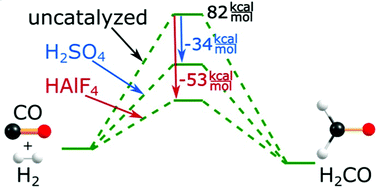
The mechanism of the CO + H2 → H2CO reaction catalyzed by acidic systems was investigated theoretically using the ab initio MP2 and CCSD(T) methods and the aug-cc-pVDZ basis set (the effects of the surrounding solvent molecules were approximated by employing the polarized continuum solvation model). Two representative acids were chosen to verify the usefulness of such catalysts in this process: sulfuric acid (as a strong mineral acid) and HAlF4 (as a superacid). Detailed mechanisms in both gas and liquid phases proceeding either along a concerted path or according to a stepwise route were investigated and discussed. The most important findings include the observation that both acids seem to catalyze the carbon monoxide hydrogenation in a qualitatively similar way but only the HAlF4 superacid is predicted to effectively reduce the activation barriers to render the whole process plausible.


 Please wait while we load your content...
Please wait while we load your content...