Synthesis of methanol from CO2 hydrogenation promoted by dissociative adsorption of hydrogen on a Ga3Ni5(221) surface†
Abstract
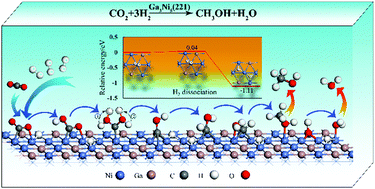
Catalytic carbon dioxide (CO2) hydrogenation to liquid fuels including methanol (CH3OH) has attracted great attention in recent years. In this work, density functional theory (DFT) calculations have been employed to study the reaction mechanisms of CO2 hydrogenation to CH3OH on Ga3Ni5(221) surfaces. The results show that all intermediates except for the O atom prefer to adsorb on Ni sites, and dissociative adsorption of hydrogen (H2) on the Ga3Ni5(221) surface is almost barrierless and highly exothermic, favoring CO2 hydrogenation. Moreover, the presence of Ga indeed enhances the dissociative adsorption of H2, and this is verified by the projected density of states (PDOS) analysis. Importantly, three possible reaction pathways based on formate (HCOO) and hydrocarboxyl (COOH) formations and reverse water gas shift (rWGS) with carbon monoxide (CO) hydrogenation have been discussed. It is found that CO2 reduction to CH3OH in these pathways prefers to occur entirely via the Langmuir–Hinshelwood (L–H) mechanism. COOH generation is the most favorable pathway because the HCOO and rWGS with CO hydrogenation pathways have high energy barriers and the resulting HCOOH intermediate in the HCOO pathway is unstable. In the COOH reaction pathway, CO2 is firstly hydrogenated to trans-COOH, followed by the formation of COH via three isomers of COHOH, its hydrogenation to trans-HCOH, and then the production of CH3OH via a CH2OH intermediate.


 Please wait while we load your content...
Please wait while we load your content...