Atomic-scale computational design of hydrophobic RE surface-doped Al2O3 and TiO2†
Abstract
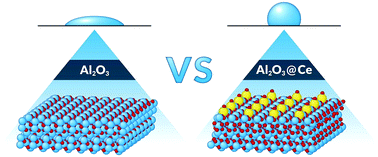
Intrinsically hydrophobic rare-earth oxides (REOs) have emerged as a robust class of ceramics for a variety of applications. Recently, the hydrophobicity of REOs has been observed experimentally and subsequently scrutinized using electronic structure density functional theory (DFT) calculations. In this work, we applied the DFT method to analyze the possibility of tuning the wettability of commonly used hydrophilic Al2O3 and TiO2 by surface doping with Ce. The calculations indicate that Ce can preferentially segregate to the surface of Al2O3 and TiO2 and form a Ce-rich oxide layer, which is stable under a wide range of oxygen chemical potentials. A remarkable increase in the water contact angle is predicted for Ce-doped Al2O3(0001), whereas the water contact angle calculated for Ce-doped TiO2(110) remains unchanged, regardless of the Ce concentration. The wetting properties of Ce-doped Al2O3 are governed by two factors: (1) the unique electronic structure of the rare-earth metal promotes hydrogen bond formation between H2O and surface oxygen; (2) significant relaxation of the surface Ce and O atoms hampers direct interaction between H2O and Al cations, preventing dissociative water adsorption. These results provide a valuable opportunity for Al2O3 surface modification, in terms of achieving hydrophobicity.


 Please wait while we load your content...
Please wait while we load your content...