Prediction of 1H NMR chemical shifts for clusters of imidazolium-based ionic liquids†
Abstract
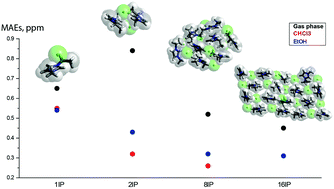
Nuclear magnetic resonance (NMR) has been widely used to elucidate the bulk structure of ionic liquids. In this work, we calculated 1H NMR chemical shifts of 1-ethyl-3-methylimidazolium (C2mim+) ionic liquids combined with various anions such as chloride (Cl), tetrafluoroborate (BF4), hexafluorophosphate (PF6), acetate (OAc), trifluoroacetate (TFA), and dicyanamide (DCA). The previously established level of theory, HF/6-311G+(3df,2p), was used for the accurate prediction of NMR chemical shifts both in gas phase and in solvents with varying dielectric constant such as CHCl3 and ethanol. The following factors affecting the predicted proton chemical shifts were considered. Firstly, ionic clusters consisting of 2, 8 and 16 ion pairs were optimized to model interionic interactions present in the bulk of ionic liquids. In larger clusters the distribution of the calculated chemical shifts of individual protons in the C2mim+ cation was examined with respect to the position of the cation in the cluster. We further confirmed that electronic properties of ionic liquids such as magnetic shielding had local nature, thus allowing us to accurately predict proton NMR chemical shifts of ionic liquids from relatively small-sized clusters. Secondly, solvent effects in single ion pairs as well as larger ionic clusters were accounted through a Conductor-like Polarisable Continuum Model (CPCM). Solvent effects generated through a dielectric constant of either chloroform or ethanol were found to be important in single ion pairs due to improved description of interionic distances. With increasing cluster size the difference between gas-phase and CPCM optimized structures became minimal, thus resulting in similar values for calculated 1H NMR chemical shifts. We also established that the model size that produced the best results for imidazolium ionic liquids strongly depended on the anion type. Strongly coordinating anions such as chloride and acetate require calculations of clusters consisting of at least 8 ion pairs, whereas weakly coordinating anions produce excellent accuracy for single ion pairs optimized in the presence of solvent. The polarity of the solvent was found to play a minor role.


 Please wait while we load your content...
Please wait while we load your content...