Ethylperoxy radical: approaching spectroscopic accuracy via coupled-cluster theory†
Abstract
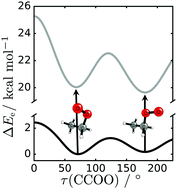
Interest in peroxy radicals derives from their central role in tropospheric and low-temperature combustion processes; however, their transient nature limits the scope of possible experimental characterization. As a result, theoretical methods (notably, coupled-cluster theory) have become indispensable in the reliable prediction of properties of such ephemeral open-shell systems. Herein, the ![[X with combining tilde]](https://www.rsc.org/images/entities/char_0058_0303.gif) and à state conformers of ethylperoxy radical (C2H5O2) have been structurally optimized at the CCSD(T)/ANO2 level of theory. Relative enthalpies at 0 K [including à ←
and à state conformers of ethylperoxy radical (C2H5O2) have been structurally optimized at the CCSD(T)/ANO2 level of theory. Relative enthalpies at 0 K [including à ← ![[X with combining tilde]](https://www.rsc.org/images/entities/char_0058_0303.gif) transition origins (T0)] are reported, incorporating CCSD(T) electronic energies extrapolated to the complete basis set limit via the focal point approach. Higher-level computations, employing basis sets as large as cc-pV5Z and post-HF methods up to CCSDT(Q), prove essential in achieving predictions to within 10 cm−1 for experimental T0; we predict 7363 and 7583 cm−1 for the trans and gauche conformers, respectively. Furthermore, predictions of
transition origins (T0)] are reported, incorporating CCSD(T) electronic energies extrapolated to the complete basis set limit via the focal point approach. Higher-level computations, employing basis sets as large as cc-pV5Z and post-HF methods up to CCSDT(Q), prove essential in achieving predictions to within 10 cm−1 for experimental T0; we predict 7363 and 7583 cm−1 for the trans and gauche conformers, respectively. Furthermore, predictions of ![[X with combining tilde]](https://www.rsc.org/images/entities/char_0058_0303.gif) state fundamental transitions incorporating CCSD(T)/ANO0 anharmonic contributions are given. For each conformer, all 21 modes were characterized, improving upon the 16 modes reported in the experimental literature, and providing predictions for the 5 remaining modes.
state fundamental transitions incorporating CCSD(T)/ANO0 anharmonic contributions are given. For each conformer, all 21 modes were characterized, improving upon the 16 modes reported in the experimental literature, and providing predictions for the 5 remaining modes.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...