Polypyrrole capacitance characteristics with different doping ions and thicknesses†
Abstract
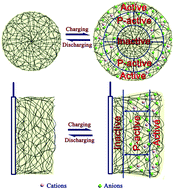
Polypyrrole films doped with Cl−, SO42− and 4-toluene sulfonic ions (PPy/Cl−, PPy/SO42−, PPy/Ts−) with different polymerization charges were prepared by electrochemical polymerization. PPy/Ts− films exhibited good electrochemical properties when their polymerization charges were 0.5 C cm−2 and 1 C cm−2. However, PPy/Cl− and PPy/SO42− films exhibited markedly larger specific capacitance than those of PPy/Ts− films when their polymerization charges increased to 2 C cm−2 or 4 C cm−2. A simple model was suggested for explaining the relation between the electrochemical properties and thickness of the PPy films. According to the model, the incremental polymerization bulk of PPy/Ts− films from 1 C cm−2 to 2 C cm−2 is “inactive”, which resulted in the reduction of the specific capacitance by half. X-ray diffraction patterns indicated that PPy/Ts− films possessed a more ordered structure, which limited ion diffusion in the PPy matrix. Therefore, the high crystallinity of the PPy film does not always mean good capacitance properties. In the cycle stability test, the PPy/Ts− film exhibited better stability, which should be closely related to its more ordered structure. It is noteworthy that we present a newly developed method to estimate the appropriate thickness of electrode materials, which is significant in the preparation of energy-storage devices.


 Please wait while we load your content...
Please wait while we load your content...