Why do zeolites induce an unprecedented electronic state on exchanged metal ions?†
Abstract
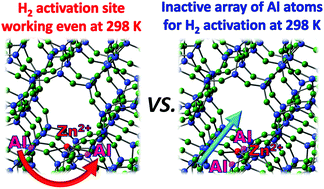
Understanding the exact position and the detailed role of the Al array in zeolites is essential for elucidating the origin of unique properties that can be derived from the metal-ion exchanged in zeolite samples and for designing zeolite materials with high efficiency in catalytic and adsorption processes. In this work, we investigate, for the first time, the important role of the Al array in the reactivity observed on the metal-ion exchanged in zeolites on the basis of the calculation method by utilizing the spontaneous heterolytic cleavage of H2 observed experimentally on the Zn2+-ion exchanged in MFI-type zeolites (Zn2+-MFI) as the model reaction. In the case of calculation, two main types of models for considering the Al positions in MFI-type zeolites were adopted: in the first type, the Al atoms with appropriate distances are aligned in the circumferential direction of the straight channel (abbreviated as a circumferentially arrayed Al–Al site); in the second type, the nearest neighbouring Al atoms with appropriate distances are directed toward the straight channel axis (abbreviated as a channel directionally arrayed Al–Al site). Results indicated that the Al-array direction governs the reactivity of Zn2+-MFI. The former type of array well explains the experimental fact that spontaneous and irreversible heterolysis of H2 takes place on Zn2+-MFI, even at room temperature, whereas the latter type of array is less reactive; high activation energy is required for the heterolytic cleavage of H2 (ca. >70 kJ mol−1). A detailed analysis of the geometric and electronic structures of a series of Zn2+-MFI models with various Al-array directions clarified the following facts: the circumferentially arrayed Al–Al site induces an inevitable environment around the Zn2+ site, with the simultaneous existence of both a Lewis acid point (coordinatively unsaturated and distorted Zn2+) and a Lewis base point (the lattice oxygen atom juxtaposed with exchanged Zn2+, which participates in the activation of H2: OjL). It is the circumferentially arrayed Al–Al atoms that confer acidic and basic nature on the metal ion and the lattice oxygen atom (OjL), and ultimately trigger the heterolytic dissociation of H2, even at 300 K.


 Please wait while we load your content...
Please wait while we load your content...