Quasi-classical trajectory studies on the full-dimensional accurate potential energy surface for the OH + H2O = H2O + OH reaction†
Abstract
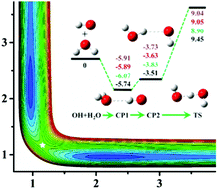
For the symmetric reaction OH + H2O → H2O + OH, ∼48 000 data points are sampled and calculated at the level of the explicitly correlated unrestricted coupled cluster method with single, double, and perturbative triple excitations with the augmented correlation-consistent polarized triple zeta basis set (CCSD(T)-F12a/AVTZ). The data set is then employed to fit the full dimensional accurate potential energy surface (PES) by using the permutation invariant polynomial-neural network (PIP-NN) method, resulting in a total root mean square error (RMSE) of 0.12 kcal mol−1. The quasi-classical trajectory (QCT) method is used to study its reaction dynamics. It has been found that the integral cross section (ICS) is increased gradually as a function of the collision energy ranging from 8 to 30 kcal mol−1 with a threshold around 8 kcal mol−1. At a collision energy of 20 kcal mol−1, detailed dynamics show that the OH product is a spectator and the differential cross section (DCS) is dominated by backward scattering with considerable contributions from sideway scattering, consistent with the direct rebound and stripping mechanisms.


 Please wait while we load your content...
Please wait while we load your content...