Local decomposition of imaginary polarizabilities and dispersion coefficients†
Abstract
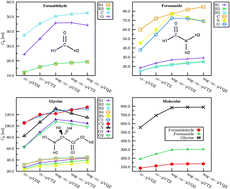
We present a new way to compute the two-body contribution to the dispersion energy using ab initio theory. By combining the complex polarization propagator method and the LoProp transformation, local contributions to the Casimir–Polder interaction is obtained. The full dispersion energy in dimer systems consisting of pairs of molecules including H2, N2, CO, CH4, pyridine, and benzene is investigated, where anisotropic as well as isotropic models of dispersion are obtained using a decomposition scheme for the dipole–dipole polarizability. It is found that the local minima structure of the π-cloud stacking of the benzene dimer is underestimated by the total molecular dispersion, but is alleviated by the inclusion of atomic interactions via the decomposition scheme. The dispersion energy in the T-shaped benzene dimer system is greatly underestimated by all dispersion models, as compared to high-level quantum calculations. The generalization of the decomposition scheme to higher order multipole polarizability interactions, representing higher order dispersion coefficients, is briefly discussed. It is argued that the incorporation of atomic C6 coefficients in new atomic force fields may have important ramifications in molecular dynamics studies of biomolecular systems.


 Please wait while we load your content...
Please wait while we load your content...