Multi-level quantum mechanics theories and molecular mechanics study of the double-inversion mechanism of the F− + CH3I reaction in aqueous solution
Abstract
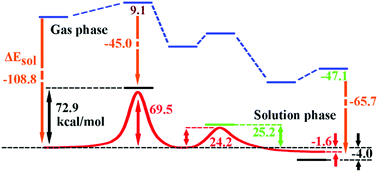
A double-inversion mechanism of the F− + CH3I reaction was discovered in aqueous solution using combined multi-level quantum mechanics theories and molecular mechanics. The stationary points along the reaction path show very different structures to the ones in the gas phase due to the interactions between the solvent and solute, especially strong hydrogen bonds. An intermediate complex, a minimum on the potential of mean force, was found to serve as a connecting-link between the abstraction-induced inversion transition state and the Walden-inversion transition state. The potentials of mean force were calculated with both the DFT/MM and CCSD(T)/MM levels of theory. Our calculated free energy barrier of the abstraction-induced inversion is 69.5 kcal mol−1 at the CCSD(T)/MM level of theory, which agrees with the one at 72.9 kcal mol−1 calculated using the Born solvation model and gas-phase data; and our calculated free energy barrier of the Walden inversion is 24.2 kcal mol−1, which agrees very well with the experimental value at 25.2 kcal mol−1 in aqueous solution. The calculations show that the aqueous solution makes significant contributions to the potentials of mean force and exerts a big impact on the molecular-level evolution along the reaction pathway.
- This article is part of the themed collection: 2017 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...